ChitopharmTM
customized and traceable
General technical information
Product range
and molecular weight
Our productrange consists of four pure and safe chitosan products with different average molecular weight but consistent quality.
Degree of
deacetylation
The degree of deacetylation is greater than 70 % (- 95 %) for our standard products. We are able to adjust the degree of deacetylation to match our customers’ needs.
Quality control
and analyses
Quality is our top priority, therefore we have established strict quality control measures. We work together with certified external labs to perform product anlyses conforming the USP/NF Monograph «Chitosan».
Endotoxins and heavy metals
Our chitosans have an extremely low endotoxin and heavy metal content. This is the result of the fact, that we never compromise on quality; it is part of every step in the production process from the raw material to pure chemicals and reliable analyses.
Microbiology
All ChitopharmTM grades conform to the requirements in the USP Monograph “Chitosan” and are absent for Staphylococcus aureus and Pseudomonas aeruginosa.
Drug Master Files
To simplify the incoporation of ChitopharmtTM into our customers’ valuable medical application, we have filed DMFs type IV at the FDA for each of our grades.
Wound-healing
ChitopharmTM has clinically proven wound-healing properties as it accelerates the healing of open wounds.
How does Chitosan improve wound-healing?
Chitosan forms a protective layer on the skin. This thin film is extremely protective but permeable for oxygen at the same time. Through stimulation of the immune response and tissue reconstruction, anti-microbial action and exudate absorption, chitosans significantly improve wound healing.
How does Chitosan stop bleeding?
Since chitosan is a natural polymer, it is an attractive material for use as a hemostatic agent. Chitosan is known to bind erythrocytes in a concentration-dependent manner that does not depend on its molecular weight. Chitosan-based material adheres to platelets and changes their shape inducing a hemostatic effect. There is in vivo evidence that chitosan-based materials can stop bleeding from arteries of relatively large diameter.
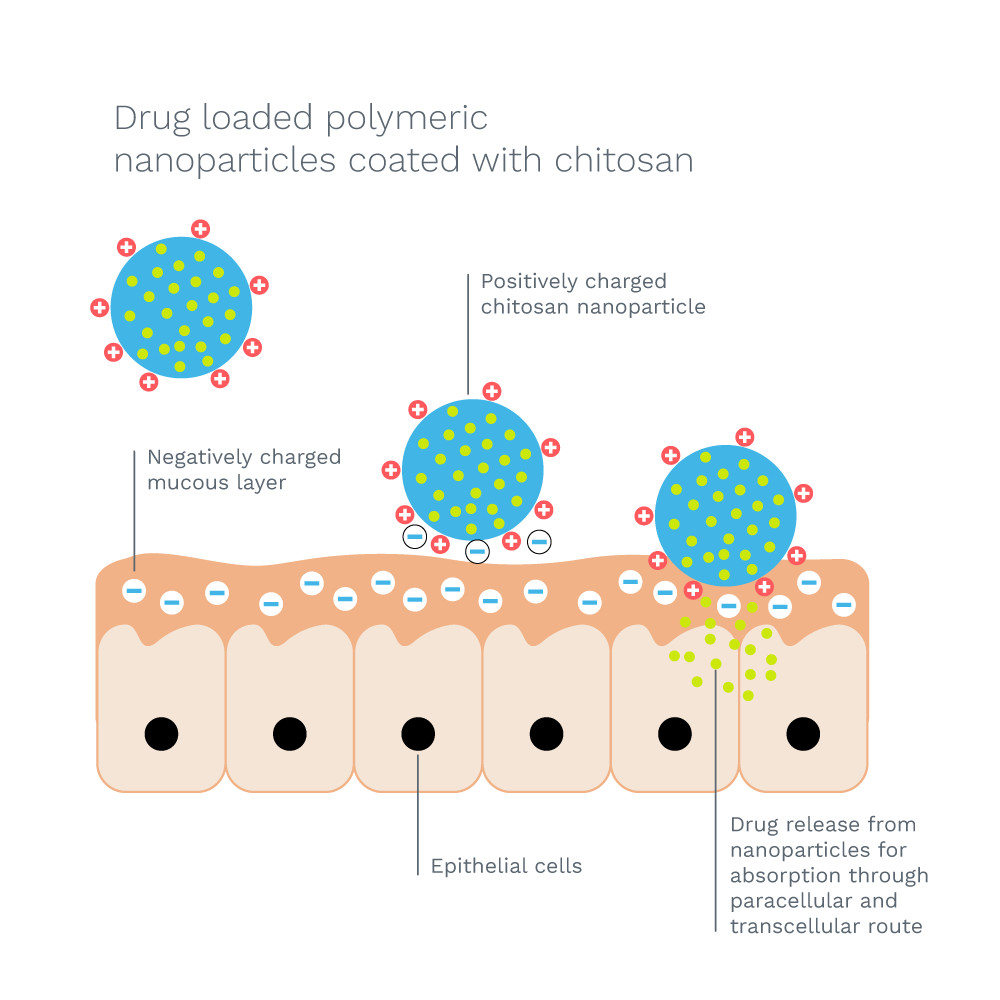
Mucoadhesive
ChitopharmTM combines muco-adhesive properties with a permeation-enhancing effect. In vivo tests show that it does not biodegrade too fast and it is therefore certified for controlled and targeted drug release.
Why is chitosan mucoadhesive?
Chitosan is positively charged and therefore binds to negatively charged surfaces such as mucosal membranes. It enhances the transport of polar drugs across epithelial surfaces. Muco-adhesive drug delivery systems can be utilized for targeted drug delivery. They will improve the retention time and controlled release of a drug.
What is a mucoadhesive drug delivery system?
Mucoadhesive substances interact with the mucus layer covering the mucosal epithelial surface.
Anti-microbial
ChitopharmTM is non-toxic, but anti-microbial, especially for yeasts, moulds, and Gram-negative bacteria. It is biocompatible and causes minimal inflammatory response and foreign body reaction.
Why is chitosan anti-microbial?
Highly deacetylated chitosans are more anti-microbial than those with a higher proportion of acetylated amino groups due to increased solubility and higher charge density. Lower pH increases the anti-microbial activity of chitosan for much the same reasons, in addition to the ‘hurdle effect’ of inflicting acid stress on the target organisms
Formulations
The incorporation of ChitopharmTM in almost any product or application is possible due to its simple processability into various forms, including hydrogels and porous scaffolds. More than 10 000 research articles in the medical field have been published on chitosan in the last 5 years.
How to dissolve Chitosan?
Chitosan is positively charged and soluble in weak acidic solutions. Chitosan powder is soluble in glycolic, acetic or lactic acid, amongst others. The solubility is dependent on the concentration and molecular weight which determines the viscosity while being little influenced by the degree of deacetylation. To increase the solubility, the solution should be heated and stirred. ChitopharmTM forms clear solutions that can be directly incorporated in your pharmaceutical production.
How to prepare Chitosan Hydrogel?
Chitosan forms gels when dispersed in a weak acid. Low (LMWC), medium (MMWC) or high (HMWC) molecular weight chitosan are usually dispersed in 2.5 % (v/w) acetic acid solution. Alternatively, 0.25, 1, 1.5, 2 and 4% acetic acid solutions can be used depending on the desired final pH of the gel. when appropriate. The usual concentration of chitosan in the acetic acid is in the range of 1-6% (w/w). The chitosan powder mixture in acetic solution is usually stirred either or by the help of stirring aid. Chitosan hydrogels need to be allowed to swell for a sufficient time (24-48 hours). It is recommended to control the stirring speed to avoid trapping of air. Bath sonication can be used to remove entrapped air bubbles. Glycerol can be added in the initial mixture when required.
